Usp 797 Clean Room Guidelines Standards

33 other professional organizations also provide guidance on specific aspects of compounding.
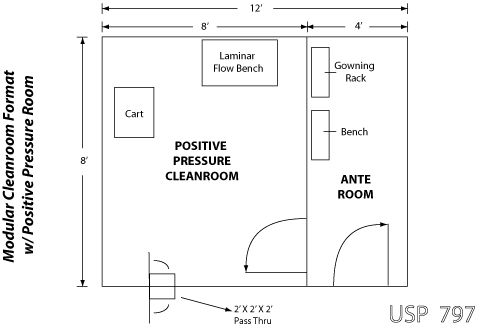
Usp 797 clean room guidelines standards. Adopt usp 797 facility engineering clean room guidelines. In june 2019 the united states pharmacopeia usp released several new and revised pharmacy compounding standards. The requirements may differ based on the state board of pharmacy. 797 pharmaceutical compounding sterile preparations.
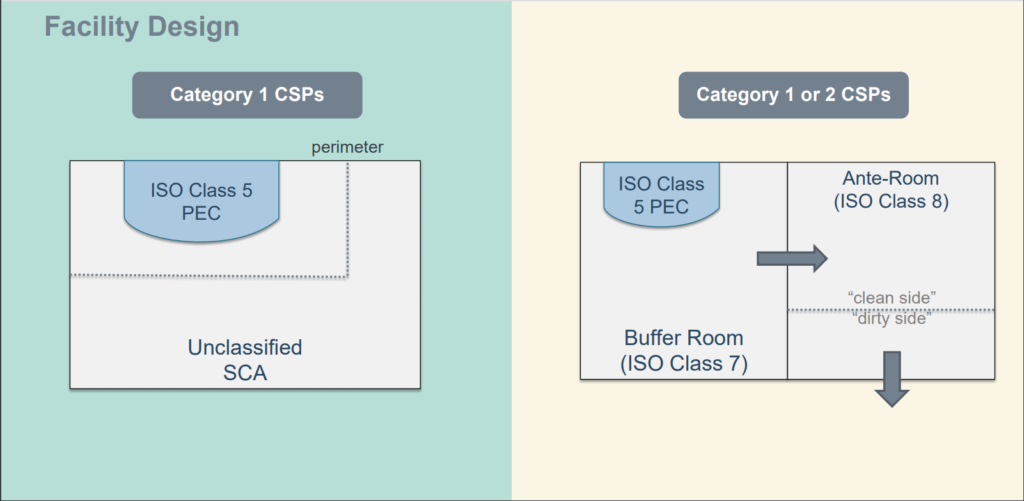
209e fs 209e class name particle count iso class u s. Our recommendations are based on a class 7 buffer zone however facilities with class 5. The entire compounding environment which includes the clean rooms and ante rooms must be disinfected on a regular basis. The revisions to usp 797 and new usp 800 standards will go into effect december 1 2019.
Our in house team of engineers architects and designers will provide assistance with the design and qualification process while our nationwide network. During this time we ve discovered the only way to make these projects successful is to not just to understand the content of. Some items like gloves hoods face masks bunny suits and shoe covers require safe and clean forward disposal after every session. In light of these appeals and in accordance with our bylaws usp is postponing the official date of 797 until further notice.
Standards for prescribing preparation. The opportunity to request further review by an appointed panel and usp has received three 3 such requests on 797. This includes floors hoods and countertops. Make sure compounding staff uses the proper cleaning agents so that all microorganisms are eliminated.
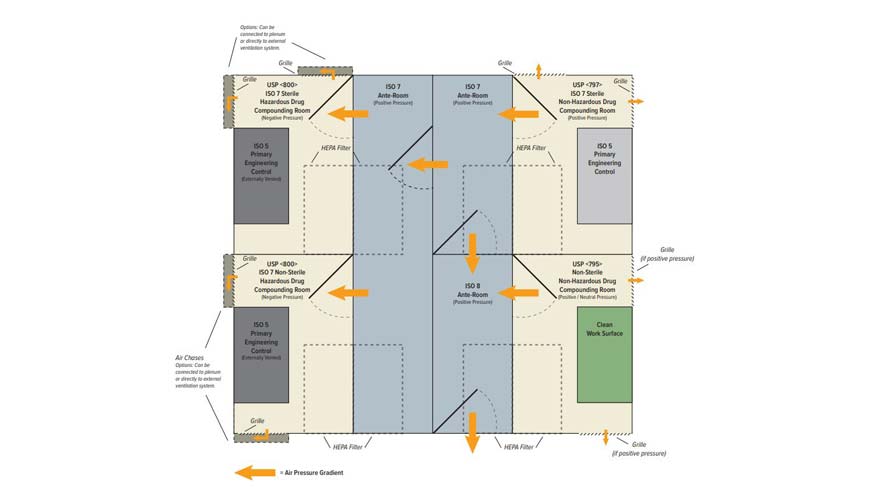
Usp 797 cleaning disinfecting guidelines. Specifically usp published revisions to general chapter 797 pharmaceutical compounding and sterile preparations and published a new general chapter 800 hazardous drugs handling in healthcare settings. Minimum standard for pharmacies in hospitals. In the interim the currently official version of 797 last revised in 2008 including the section.
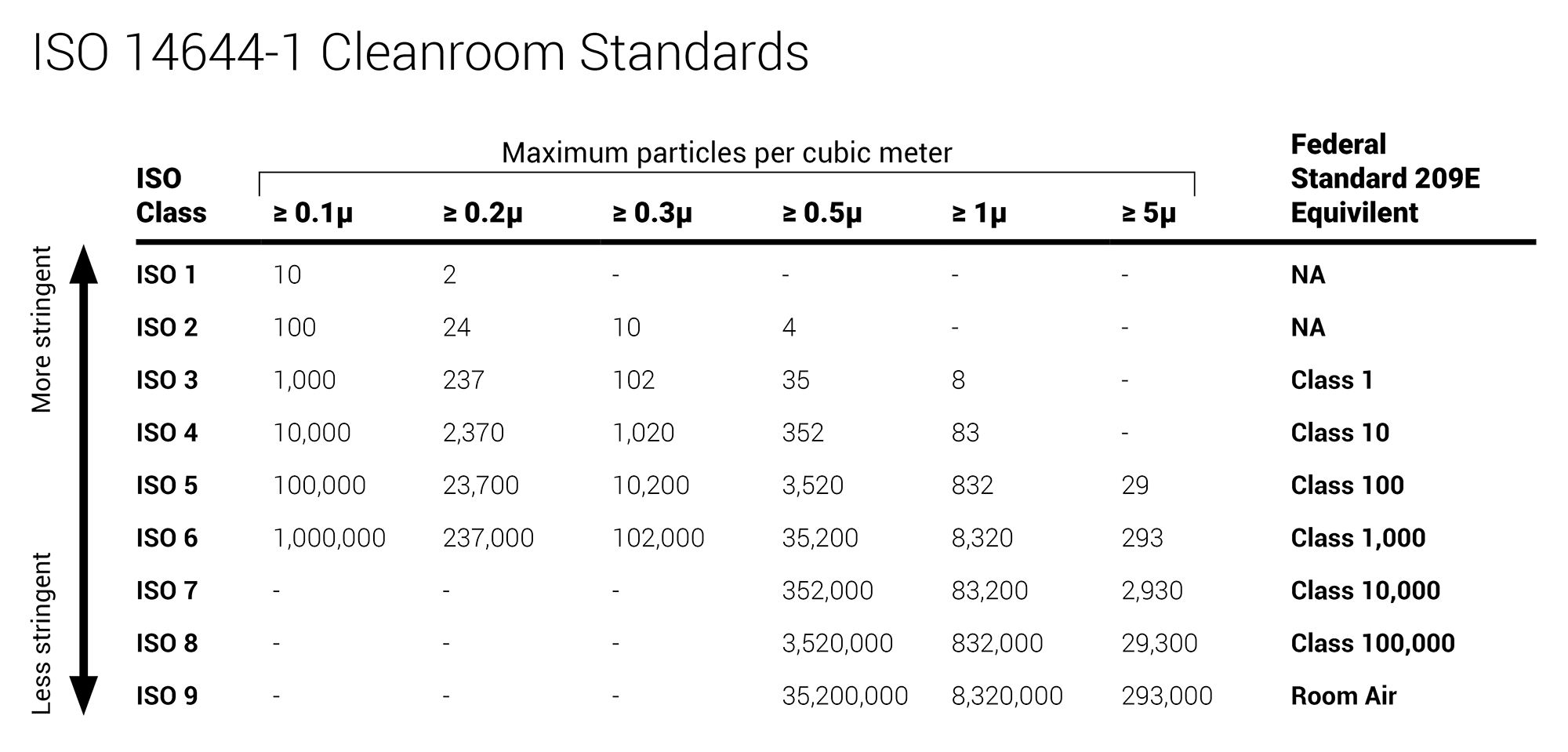
Usp 797 standards require consideration for proper gowning and personal protective equipment ppe. Iso classification of particulate matter in room air limits are in particles of 0 5 μm and larger per cubic meter current iso and cubic feet former federal standard no. Achieving pharmacy flow with usp 797 and usp 800 standards october 10 2019 pharmacies and compounding laboratories are a hot topic in the health care world as the us pharmacopeial convention s usp revised guidelines necessitate new pharmacy design to meet usp 797 and usp 800 compliance. Over the course of 2018 the henderson engineers team of experts has evaluated nearly a hundred facilities across the country to prepare their pharmacies for this deadline.
Fs 209e iso m. Chapter outlines minimal cleaning and disinfecting requirements for the typical sterile compounding pharmacy environment. Recommended usp 797 cleaning disinfecting decontaminating for sterile compounding.